Classify the Statements About Redox Reactions as True or False.
Accompany all chemical changes c. If there are no changes in the oxidation state of the reactants or products of a particular reaction that reaction is not a redox reaction.

Ncert Exemplar Class 11 Chemistry Solutions Chapter 8 Redox Reactions
Szatement TrueFalse In Ka Kb and Kw reactions a species is reacting with water.

. Textbook solution for Introductory Chemistry. True False Answer Bank A reducing agent gets oxidized as it reacts. A reducing agent gets oxidized as it reacts.
1 Answer anor277 Mar 23 2018. If there are no changes in the oxidation state of the reactants or products of a particular reaction that reaction is not a redox reaction. In the redox reaction Fe3 Co2 Fe2 Co3 Fe3 is the reducing agent and Co2 is the oxidizing agent.
Szatement TrueFalse In Ka Kb and Kw reactions a species is reacting with water. Classify each off following statements as to or falls. In the redox reaction Fe3 Co2 Fe2 Co2 Fe3 is the reducing agent and Co2 is the oxidizing agent.
So option 4 is true. True or False Oxidation reactions are the principal source of energy on Earth. The loss of electrons from a substance involved in a redox reaction.
Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. Which of the following sequences of T and F is correct for given statements. Reducing agents may accept H ions.
True or False Not all oxidation reactions are accompanied by reduction reactions. True False Answer Bank If something is reduced it is formally losing electrons. D Hypothetically nitrate NO3 would make a better terminal electron acceptor than NAD E Nitrate NO3 is a stronger electron acceptor.
Here T stands for true and F stands for false statements i Reducing agents lower the oxidation number of an element in a given substance. Classify the statements about redox reactions as true or false. Which statement is true regarding redox reaction.
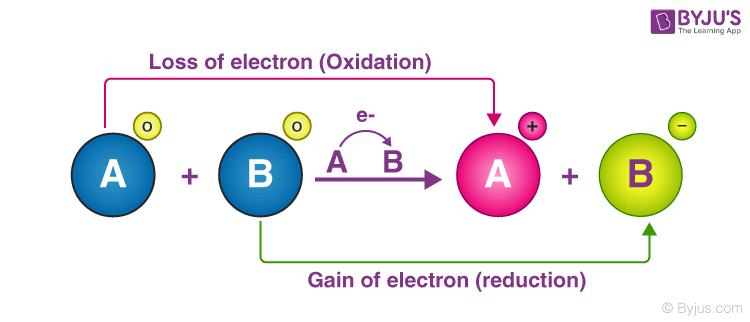
Reactions that involve the transfer of electrons are referred to as oxidation-reduction reaction or redox reactions. B NADH is a stronger electron donor than nitrite NO2 C NADH oxidation releases energy. Co3 Fe3 is the reducing agent and Co² is the oxidizing agent.
A method of balancing a redox equation by comparing the increases and decreases in oxidation numbers. Result in a change in the oxidation states of the species involved e. The term redox is a short form of reduction-oxidation.
A positive or negative number assigned to an atom to indicate its degree of oxidation or reduction. Electrons are in terms off the oxidation number. Which statement correctly describes a redox reaction.
The oxidation half-reaction and the reduction half-reaction occur simultaneously. In the redox reaction Fe Co - Fe2 Co Fe is the reducing. Oxidizing agents may accept H ions.
So this question is from the chapter oxidation a reduction a Redox reaction and the concept behind this question is the redox reaction. Redox reactions may involve the transfer of hydrogen ions H. These reagents are also called reductants ii Reducing agents are acceptors of electrons.
An Active Learning Approach 6th Edition Mark S. Oxidation and reduction a. If something is reduced it is formally losing electrons.
Which statement is false regarding the redox reactions shown above. The oxidation half-reaction occurs before the reduction half-reaction. Which of the following statements is are true.
All the redox reactions can be broken down into two different processes a reduction process and an oxidation process. What is the lewis structure for hcn. True False Oxidizing agents can convert CO into CO2.
Describe the loss and gain of electrons respectively d. If something is oxidized it is formally losing electrons. And option 5 is FALSE by this reason.
In a redox reaction one element has a complete or partial loss of electrons oxidation therefore another element must have a complete or partial gain of electrons reduction. If something is oxidized it is formally losing electrons. We have step-by-step solutions for your textbooks written by Bartleby experts.
So a redox reaction is direction in which both oxidation and reduction can take place. These redox reactions occur in covalent and ionic compounds. Cracolice Chapter 19 Problem 54E.
So oxidation is the laws off. Oxidation is Loss Reduction is Gain True or False. Science Chemistry QA Library Classify each statement as true or false.
Statements that can be considered as a true statement as regards redox reactions are. A reducing agent gets oxidized as it reacts. True What does OIL RIG stand for.
How is vsepr used to classify molecules. A It requires energy to reduce nitrate NO3. Oxidizing agents accept electrons.
A molecule that has gained H atoms is said to be reduced. Redox reactions have pairs. True False Answer Bank A reducing agent gets oxidized as it reacts.
In the redox reaction Fe3 Co² Fe. Cannot occur independently of each other b. If there are no changes in the oxidation state of the reactants or products of a particular reaction that reaction is not a redox reaction.

Ncert Exemplar Class 11 Chemistry Solutions Chapter 8 Redox Reactions

Ncert Exemplar Class 11 Chemistry Solutions Chapter 8 Redox Reactions

Ncert Exemplar Class 11 Chemistry Solutions Chapter 8 Redox Reactions
No comments for "Classify the Statements About Redox Reactions as True or False."
Post a Comment